Courtesy : Bachelor of Science Biology (CBZ) – Chemistry, Botany, Zoology
Chemical basis
Atoms and molecules
Further information: Chemistry
All organisms are made up of chemical elements; oxygen, carbon, hydrogen, and nitrogen account for 96%[further explanation needed] of all organisms, with calcium, phosphorus, sulfur, sodium, chlorine, and magnesium constituting essentially all the remainder. Different elements can combine to form compounds such as water, which is fundamental to life. Biochemistry is the study of chemical processes within and relating to living organisms. Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including molecular synthesis, modification, mechanisms, and interactions. # ISO certification in India
Water

Model of hydrogen bonds (1) between molecules of water
See also: Planetary habitability, Circumstellar habitable zone, and Water distribution on Earth
Life arose from the Earth’s first ocean, which was formed approximately 3.8 billion years ago. Since then, water continues to be the most abundant molecule in every organism. Water is important to life because it is an effective solvent, capable of dissolving solutes such as sodium and chloride ions or other small molecules to form an aqueous solution. Once dissolved in water, these solutes are more likely to come in contact with one another and therefore take part in chemical reactions that sustain life.# ISO certification in India
In terms of its molecular structure, water is a small polar molecule with a bent shape formed by the polar covalent bonds of two hydrogen (H) atoms to one oxygen (O) atom (H2O). Because the O–H bonds are polar, the oxygen atom has a slight negative charge and the two hydrogen atoms have a slight positive charge. This polar property of water allows it to attract other water molecules via hydrogen bonds, which makes water cohesive. Surface tension results from the cohesive force due to the attraction between molecules at the surface of the liquid. Water is also adhesive as it is able to adhere to the surface of any polar or charged non-water molecules.# ISO certification in India
Water is denser as a liquid than it is as a solid (or ice). This unique property of water allows ice to float above liquid water such as ponds, lakes, and oceans, thereby insulating the liquid below from the cold air above. The lower density of ice compared to liquid water is due to the lower number of water molecules that form the crystal lattice structure of ice, which leaves a large amount of space between water molecule. In contrast, there is no crystal lattice structure in liquid water, which allows more water molecules to occupy the same amount of volume.# ISO certification in India
Water also has the capacity to absorb energy, giving it a higher specific heat capacity than other solvents such as ethanol. Thus, a large amount of energy is needed to break the hydrogen bonds between water molecules to convert liquid water into water vapor.
As a molecule, water is not completely stable as each water molecule continuously dissociates into hydrogen and hydroxyl ions before reforming into a water molecule again. In pure water, the number of hydrogen ions balances (or equals) the number of hydroxyl ions, resulting in a pH that is neutral.# ISO certification in India
Organic compounds
Further information: Organic chemistry
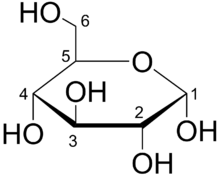
Organic compounds such as glucose are vital to organisms.
Organic compounds are molecules that contain carbon bonded to another element such as hydrogen. With the exception of water, nearly all the molecules that make up each organism contain carbon. Carbon can form covalent bonds with up to four other atoms, enabling it to form diverse, large, and complex molecules. For example, a single carbon atom can form four single covalent bonds such as in methane, two double covalent bonds such as in carbon dioxide (CO2), or a triple covalent bond such as in carbon monoxide (CO). Moreover, carbon can form very long chains of interconnecting carbon–carbon bonds such as octane or ring-like structures such as glucose.
The simplest form of an organic molecule is the hydrocarbon, which is a large family of organic compounds that are composed of hydrogen atoms bonded to a chain of carbon atoms. A hydrocarbon backbone can be substituted by other elements such as oxygen (O), hydrogen (H), phosphorus (P), and sulfur (S), which can change the chemical behavior of that compound. Groups of atoms that contain these elements (O-, H-, P-, and S-) and are bonded to a central carbon atom or skeleton are called functional groups. There are six prominent functional groups that can be found in organisms: amino group, carboxyl group, carbonyl group, hydroxyl group, phosphate group, and sulfhydryl group.
In 1953, the Miller-Urey experiment showed that organic compounds could be synthesized abiotically within a closed system mimicking the conditions of early Earth, thus suggesting that complex organic molecules could have arisen spontaneously in early Earth (see abiogenesis)



