Colloids- Colloids are a type of mixture in which tiny particles of one substance are dispersed evenly throughout another substance. These particles are typically between 1 and 1000 nanometers in size, which is larger than individual molecules but smaller than visible particles. Colloidal systems can be found in various states, including solid, liquid, and gas dispersed in another solid, liquid, or gas. The three primary components of a colloid are:
- Dispersed Phase: This refers to the tiny particles or droplets that are dispersed throughout the medium. These particles can be solid, liquid, or gas and are often referred to as colloidal particles.
- Medium or Continuous Phase: This is the substance in which the dispersed phase is suspended. It can also be a solid, liquid, or gas, depending on the type of colloid. The continuous phase surrounds the colloidal particles.
- Interface: The interface is the boundary or surface that separates the dispersed phase from the continuous phase. This interface plays a crucial role in stabilizing the colloid and preventing the colloidal particles from settling out.
Colloids exhibit several distinctive properties:
- Tyndall Effect: When a beam of light passes through a colloid, it scatters the light, making the beam visible. This phenomenon is known as the Tyndall effect and is used to distinguish colloids from solutions, where light passes through without scattering.
- Brownian Motion: Colloidal particles exhibit random, continuous motion in the medium due to collisions with molecules of the continuous phase. This motion, known as Brownian motion, is a result of the kinetic energy of the particles and helps prevent them from settling.
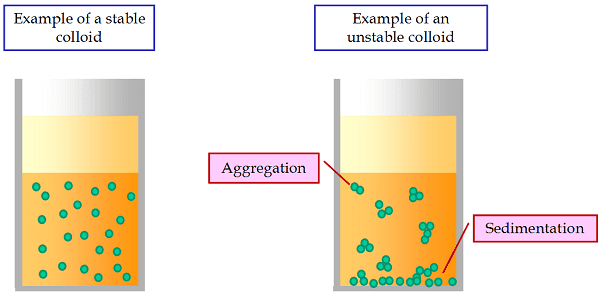
- Stability: Colloids can be stable or unstable. Stable colloids have particles that remain dispersed over time, while unstable colloids tend to coagulate or flocculate, causing the particles to come together and settle out.
- Particle Size: Colloidal particles are often very small, which provides them with a large surface area relative to their volume. This large surface area gives colloids unique properties, such as the ability to adsorb other substances.
Common examples of colloids include:
- Aerosols: Colloidal systems in which tiny solid or liquid particles are dispersed in a gas. Examples include smoke and mist.
- Emulsions: Colloidal systems consisting of tiny liquid droplets dispersed in another immiscible liquid. Examples include mayonnaise and milk.
- Sols: Colloidal systems in which solid particles are dispersed in a liquid. Examples include paint and ink.
- Foams: Colloidal systems where gas bubbles are dispersed in a liquid or solid. Examples include whipped cream and foam insulation.
Colloids have many practical applications in various fields, including food science, pharmaceuticals, cosmetics, and materials science, due to their unique properties and ability to stabilize and disperse substances.
What is Colloids
Colloids are a type of heterogeneous mixture composed of two or more substances that are dispersed on a microscopic scale but do not dissolve into each other. In a colloid, one substance is evenly dispersed as tiny particles or droplets within another substance. These particles are larger than individual molecules but smaller than visible particles, typically falling in the size range of 1 to 1000 nanometers. Colloidal systems can exist in various states, including solid, liquid, or gas dispersed within another solid, liquid, or gas.
The key components of a colloid are:
- Dispersed Phase: This is the substance that exists in the form of small particles or droplets and is dispersed throughout the medium. The dispersed phase can be a solid, liquid, or gas, and it is often referred to as colloidal particles.
- Continuous Phase: This is the medium or substance in which the dispersed phase is suspended. The continuous phase can also be a solid, liquid, or gas, depending on the type of colloid. It surrounds and suspends the colloidal particles.
- Interface: The interface is the boundary or surface that separates the dispersed phase from the continuous phase. It plays a crucial role in stabilizing the colloid, preventing the colloidal particles from settling or coagulating.
Colloids exhibit several distinctive properties and behaviors, including:
- Tyndall Effect: When a beam of light passes through a colloid, it scatters the light, making the beam visible. This phenomenon is known as the Tyndall effect and is a way to detect the presence of colloidal particles.
- Brownian Motion: Colloidal particles undergo continuous, random motion in the medium due to collisions with molecules of the continuous phase. This motion, called Brownian motion, helps prevent the particles from settling.
- Stability: Colloids can be either stable or unstable. Stable colloids maintain their dispersed state over time, while unstable colloids may undergo coagulation or flocculation, causing the particles to come together and settle.
- Large Surface Area: Colloidal particles have a high surface area relative to their volume, which makes them effective in adsorbing other substances and participating in chemical reactions.
Examples of common colloids include:
- Aerosols: Tiny solid or liquid particles suspended in a gas. Examples include smoke and fog.
- Emulsions: Liquid droplets dispersed in another immiscible liquid. Examples include mayonnaise and salad dressing.
- Sols: Solid particles dispersed in a liquid. Examples include ink and paint.
- Foams: Gas bubbles dispersed in a liquid or solid. Examples include whipped cream and foam insulation.
Colloids find applications in various industries, including food and beverage, pharmaceuticals, cosmetics, and materials science, due to their unique properties and ability to stabilize and disperse substances.
Who is Required Colloids
The term “Colloids” typically refers to a class of mixtures rather than an individual person or entity. Colloids are a type of heterogeneous mixture composed of two or more substances where one substance is dispersed in another on a microscopic scale. These substances can be in the form of small particles or droplets that are larger than individual molecules but smaller than visible particles, typically falling in the size range of 1 to 1000 nanometers.
Colloids are not associated with a specific individual or entity; rather, they are a scientific concept and category used to describe certain types of mixtures that have unique properties and characteristics. If you have a specific question about colloids or a particular aspect you’d like to know more about, please provide additional details, and I’ll be happy to assist you further.
When is Required Colloids
I apologize, but your question is still unclear. The term “Required Colloids” doesn’t appear to refer to a recognized concept, event, or specific time. It’s possible there might be a misunderstanding or typo in your question. If you could provide more context or clarify your question, I would be happy to assist you further. Please specify what you are looking for or trying to understand related to colloids, and I’ll do my best to provide you with relevant information.
If you have a specific question about colloids or if there’s a particular aspect of colloids you’d like to know more about, please provide more details, and I’ll be happy to assist you further.
Where is Required Colloids
Colloids can be found in various places and settings, depending on their specific applications and natural occurrences. Colloidal systems exist in both natural and artificial environments. Here are some examples of where you can encounter colloids:
- Natural Environments:
- Fog and Mist: Suspended tiny water droplets in the air.
- Clouds: Collections of water droplets or ice crystals in the atmosphere.
- Smoke: Particles from burning materials suspended in the air.
- River Water: Suspended particles, such as silt or clay, in flowing water.
- Milk: Fat globules dispersed in water.
- Blood Plasma: Protein molecules and other substances suspended in blood.
- Soil: Solid particles and organic matter suspended in groundwater.
- Industrial and Laboratory Settings:
- Ink: Pigment particles dispersed in a liquid solvent.
- Paint: Pigment particles in a liquid medium.
- Cosmetics: Creams, lotions, and makeup products often contain colloidal systems.
- Pharmaceuticals: Some drugs and drug formulations utilize colloidal systems.
- Food and Beverage Industry: Colloids can be found in products like mayonnaise, salad dressing, and beverages.
- Geological and Environmental Processes:
- Clay Deposits: Colloidal particles can be found in certain types of clay.
- Natural Groundwater: Colloids can affect the movement of contaminants in groundwater.
- Biological Systems:
- Cells: Colloidal structures can exist within cells, such as organelles.
- DNA and Proteins: These biological macromolecules have colloidal properties.
In summary, colloids can be encountered in various natural and human-made contexts, depending on the substances involved and the conditions under which they are dispersed. They are a common feature of many everyday materials and processes.
How is Required Colloids
Colloids are created when tiny particles or droplets of one substance are dispersed evenly throughout another substance. The process of forming colloids involves several mechanisms and can occur in various ways, depending on the type of colloid and the substances involved. Here’s how colloids can be formed:
- Dispersion: The primary mechanism for forming colloids is the dispersion of one substance into another. This can happen through various means, including:
- Mechanical Dispersion: Mechanical force, such as stirring or shaking, can break down larger particles into smaller ones, dispersing them throughout the medium. For example, shaking a salad dressing can create an emulsion where oil droplets disperse in vinegar.
- Chemical Reaction: Some chemical reactions can produce colloidal particles as intermediates. These particles may remain dispersed if the conditions are suitable. For example, the synthesis of certain nanoparticles.
- Condensation: In some cases, vapor or gas can condense into tiny droplets, creating aerosol colloids. This often happens in natural processes, such as the formation of fog or mist.
- Stabilization: Colloids are often stabilized to prevent the dispersed particles from coagulating or settling. Various mechanisms can contribute to colloidal stability:
- Electrostatic Repulsion: Particles may carry electrical charges that cause them to repel each other, preventing aggregation.
- Steric Hindrance: Molecules or polymers at the particle surface can physically block or hinder particles from coming into close contact.
- Adsorption: Molecules from the surrounding medium can adsorb onto the surface of colloidal particles, providing a stabilizing layer.
- Hydration: Water molecules can form a layer around colloidal particles, helping to prevent aggregation.
The resulting colloids can have a wide range of properties, depending on the size, shape, and composition of the particles, as well as the nature of the medium and the forces involved. Colloidal systems are prevalent in nature and are also commonly used in various industries and applications, including food and beverage production, pharmaceuticals, cosmetics, and materials science.
Case Study on Colloids
Colloids in Food Products
Background: A food processing company is looking to improve the texture and stability of its salad dressing products. The company has been experiencing issues with separation of oil and vinegar in their vinaigrette dressings, resulting in an unappealing appearance and inconsistent taste for consumers. They are seeking a solution to create a stable emulsion, which is a common colloidal system.
Challenges:
- Emulsion Stability: The primary challenge is to create an emulsion of oil and vinegar that remains stable over time, preventing phase separation.
- Texture: The company wants to achieve a smooth and creamy texture for their vinaigrette dressing, which requires proper dispersion of the oil droplets.
Solution: To address these challenges, the food processing company decides to use colloidal science principles to improve their salad dressing products.
- Emulsifying Agents: They select appropriate emulsifying agents (surfactants) that can reduce the surface tension between oil and vinegar, allowing them to mix more effectively. Common emulsifiers used in the food industry include lecithin, egg yolks, and certain proteins.
- Mechanical Mixing: They invest in specialized mixing equipment that provides uniform and consistent shear forces to disperse the oil droplets evenly throughout the vinegar. This helps create a stable emulsion.
- Particle Size Control: Controlling the size of the oil droplets is critical. They use homogenization techniques to break down large oil droplets into smaller, more uniform sizes. Smaller droplets increase stability and enhance the texture of the dressing.
- pH and Ingredient Selection: They adjust the pH of the formulation to ensure the emulsifying agents are most effective. Additionally, they carefully choose ingredients to ensure compatibility and long-term stability.
Results: By implementing these strategies, the food processing company successfully improves the stability and texture of their vinaigrette dressings. The colloidal principles of emulsion creation and stabilization are key to achieving the desired product quality. As a result, their salad dressings no longer experience phase separation, and customers enjoy a consistent, creamy-textured product.
Conclusion: This case study highlights the importance of understanding colloidal science in the food industry, where colloids play a crucial role in achieving desired product properties. By applying knowledge of emulsions and colloidal stability, companies can enhance the quality and shelf life of their food products while meeting consumer preferences.
White paper on Colloids
Creating a full white paper on colloids would require a more extensive and in-depth analysis than can be provided here, but I can offer you a structured outline and some key points to include in such a document. You can then use this as a starting point to develop a comprehensive white paper on the topic of colloids. Here’s an outline and some content suggestions:
Title: Understanding Colloids: Properties, Applications, and Impact
Table of Contents
- Abstract
- Brief summary of the white paper’s content.
- Introduction
- Explanation of what colloids are and why they are significant.
- Purpose and scope of the white paper.
- What Are Colloids?
- Definition of colloids.
- Distinction from other types of mixtures (solutions and suspensions).
- Size range and characteristics of colloidal particles.
- Types of Colloids
- Classification based on the phases involved (e.g., sols, emulsions, aerosols).
- Examples of each type with real-world applications.
- Properties of Colloids
- Overview of key properties:
- Tyndall effect.
- Brownian motion.
- Stability and coagulation.
- Surface area and adsorption.
- How these properties differ from solutions and suspensions.
- Overview of key properties:
- Formation of Colloids
- Mechanisms for creating colloids:
- Mechanical dispersion.
- Chemical reactions.
- Condensation.
- Stabilization mechanisms:
- Electrostatic repulsion.
- Steric hindrance.
- Adsorption.
- Hydration.
- Mechanisms for creating colloids:
- Applications of Colloids
- Real-world examples across various industries:
- Food and beverage.
- Pharmaceuticals.
- Cosmetics.
- Materials science.
- Environmental science.
- How colloids improve product quality and performance.
- Real-world examples across various industries:
- Measurement and Characterization
- Techniques for characterizing colloidal systems:
- Electron microscopy.
- Dynamic light scattering.
- Zeta potential measurements.
- Spectroscopy.
- Importance of accurate characterization in research and industry.
- Techniques for characterizing colloidal systems:
- Challenges and Issues
- Common challenges in dealing with colloids:
- Stability issues.
- Agglomeration.
- Environmental impact.
- Strategies for addressing these challenges.
- Common challenges in dealing with colloids:
- Future Trends
- Emerging applications and research areas in colloidal science.
- Sustainability and green technologies.
- Advances in colloidal drug delivery systems.
- Conclusion
- Recap of key points.
- The enduring significance of colloids in science and industry.
- References
- Cite relevant academic sources, research papers, and publications.
This outline provides a comprehensive structure for a white paper on colloids. You can expand each section with detailed information, case studies, graphs, and data to create a comprehensive and informative document on the topic. Additionally, you can include visuals such as diagrams or illustrations to enhance the understanding of colloidal concepts.






