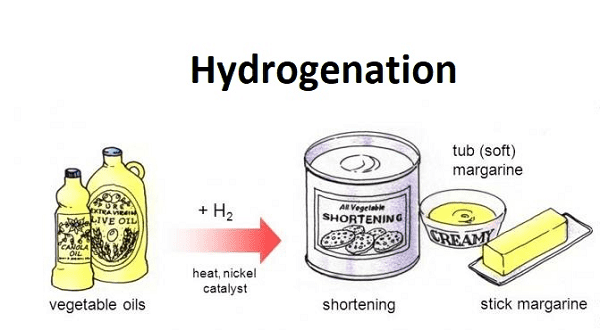
Hydrogenation- Hydrogenation is a chemical reaction in which hydrogen (H2) is added to a compound, typically an unsaturated organic compound, to reduce its degree of unsaturation. This process is commonly used in various industries and applications, including the food industry, the petrochemical industry, and the production of various chemicals and fuels.
The most well-known application of hydrogenation is in the food industry, where it is used to convert liquid vegetable oils into solid fats, such as margarine or shortening. This process helps improve the texture, flavor, and shelf life of food products. The hydrogenation of unsaturated fats involves adding hydrogen molecules to the carbon-carbon double bonds present in the fatty acid chains, converting them into single bonds. This results in the formation of saturated fats, which are more stable at room temperature.
There are two main types of hydrogenation processes:
- Partial Hydrogenation: In partial hydrogenation, not all of the double bonds in the unsaturated compound are converted to single bonds. This process is commonly used in the food industry to produce partially hydrogenated oils, but it can also produce trans fats, which have been associated with health issues when consumed in excess.
- Complete Hydrogenation: Complete hydrogenation involves converting all of the double bonds in the unsaturated compound into single bonds. This is typically done in industrial settings to produce saturated fats or to hydrogenate other organic compounds for various chemical processes.
Hydrogenation reactions typically require the presence of a catalyst, such as a metal catalyst like nickel, palladium, or platinum, to facilitate the reaction. The reaction is carried out at elevated temperatures and pressures, and hydrogen gas is bubbled through the liquid or exposed to the solid reactant.
In addition to its use in food production, hydrogenation is also used in the petrochemical industry to convert unsaturated hydrocarbons into more saturated forms, as well as in the synthesis of various chemicals and pharmaceuticals. It is an important tool in organic chemistry for modifying the properties of organic molecules and is widely used in industrial processes to create a wide range of products.
What is Hydrogenation
Hydrogenation is a chemical reaction in which hydrogen atoms are added to a compound, typically a molecule with carbon-carbon double or triple bonds, to saturate these bonds and convert them into single bonds. This process is often used in the context of organic chemistry, where it plays a crucial role in the modification and synthesis of various organic compounds.
Key points about hydrogenation include:
- Saturation: The primary goal of hydrogenation is to saturate unsaturated compounds. Unsaturated compounds contain double or triple bonds between carbon atoms, which are relatively reactive. By adding hydrogen, these reactive bonds are converted into stable single bonds, resulting in a saturated compound.
- Catalyst: Hydrogenation reactions typically require a catalyst to facilitate the reaction. Common catalysts include metals like platinum, palladium, or nickel. These catalysts help break the hydrogen molecules apart and facilitate their addition to the unsaturated compound.
- Hydrogen Gas: Hydrogenation involves the use of hydrogen gas (H2), which is bubbled through or otherwise introduced into the reaction mixture. The hydrogen gas provides the hydrogen atoms needed to react with the unsaturated compound.
- Temperature and Pressure: Hydrogenation reactions are often carried out at elevated temperatures and pressures, which can vary depending on the specific reaction and catalyst used. These conditions are necessary to promote the reaction and ensure it occurs efficiently.
- Industrial Applications: Hydrogenation has numerous industrial applications. In the food industry, it is used to convert liquid vegetable oils into solid fats, such as margarine, to improve texture and shelf life. In the petrochemical industry, it is used to refine crude oil and convert unsaturated hydrocarbons into saturated ones. It is also employed in the production of chemicals, plastics, and pharmaceuticals.
- Partial vs. Complete Hydrogenation: Hydrogenation can be partial or complete. In partial hydrogenation, not all of the double or triple bonds in the compound are saturated, leading to the formation of partially hydrogenated compounds. Complete hydrogenation converts all unsaturated bonds into saturated ones.
- Trans Fats: One important consideration with partial hydrogenation is the formation of trans fats, which are unhealthy when consumed in excess. As a result, there has been a push to reduce or eliminate the use of partially hydrogenated oils in the food industry.
In summary, hydrogenation is a chemical process that involves adding hydrogen atoms to unsaturated compounds to reduce their reactivity and create more stable saturated compounds. It has a wide range of applications in various industries and is a fundamental tool in organic chemistry for synthesizing and modifying organic molecules.
Who is Required Hydrogenation
The process of hydrogenation is used in various industries and for a wide range of applications. Different industries and sectors require hydrogenation for different purposes. Here are some of the industries and areas where hydrogenation is commonly required:
- Food Industry: The food industry extensively uses hydrogenation to convert liquid vegetable oils into solid fats. This process is used to produce products like margarine, shortening, and certain types of baked goods. It improves the texture, taste, and shelf life of these food products.
- Petrochemical Industry: In the petrochemical industry, hydrogenation is employed in refining processes to convert unsaturated hydrocarbons found in crude oil into more stable, saturated hydrocarbons. This helps in producing cleaner fuels and other petroleum-derived products.
- Chemical Industry: Hydrogenation is used in the chemical industry for various purposes, including the reduction of functional groups in organic compounds. It is often employed in the synthesis of pharmaceuticals, specialty chemicals, and fine chemicals.
- Biodiesel Production: Hydrogenation can be used in the production of biodiesel to improve its properties and stability. Unsaturated components in vegetable oils and animal fats can be hydrogenated to make them more suitable as biodiesel feedstocks.
- Oil and Fat Processing: Beyond the food industry, hydrogenation is used in the processing of oils and fats for industrial purposes, such as in the production of soaps, cosmetics, and industrial lubricants.
- Polymer Industry: Hydrogenation can be used to modify polymers, particularly in the production of elastomers and plastics. It helps in improving the properties of these materials, including their stability and resistance to heat and oxidation.
- Pharmaceutical Industry: Pharmaceutical manufacturers may use hydrogenation in the synthesis of specific drug compounds or in the reduction of functional groups to create new pharmaceutical intermediates.
- Environmental Remediation: In some cases, hydrogenation can be used for environmental remediation, such as the removal of contaminants or pollutants from soil and water.
- Chemical Research: Hydrogenation is a fundamental tool in chemical research for studying reaction mechanisms and for reducing or saturating specific functional groups in organic molecules.
The specific requirements for hydrogenation can vary widely depending on the industry and application. Different types of catalysts, reaction conditions (temperature and pressure), and equipment may be used to achieve the desired results. Additionally, there is an ongoing effort in some industries to develop more sustainable and environmentally friendly hydrogenation processes.
When is Required Hydrogenation
Hydrogenation is required or used in various situations and applications, depending on the specific needs of different industries and processes. Here are some common situations when hydrogenation is required or beneficial:
- Food Production: Hydrogenation is used in the food industry to convert liquid vegetable oils into solid fats. This is required for products like margarine, shortening, and certain baked goods to achieve the desired texture, stability, and shelf life.
- Petrochemical Refining: Hydrogenation is employed in the refining of crude oil in the petrochemical industry. It is used to remove or reduce the levels of unsaturated hydrocarbons in crude oil, leading to the production of cleaner and more stable petroleum-based products, including gasoline and diesel fuel.
- Chemical Synthesis: In the chemical industry, hydrogenation is required for various chemical synthesis processes. It is used to reduce or saturate specific functional groups in organic compounds, enabling the production of various chemicals, pharmaceuticals, and specialty products.
- Biodiesel Production: Hydrogenation can be used in the production of biodiesel to improve the properties and stability of the fuel. Unsaturated components in vegetable oils and animal fats are hydrogenated to enhance the biodiesel’s quality.
- Polymer Modification: The polymer industry utilizes hydrogenation to modify polymers, particularly in the production of elastomers and plastics. This process enhances the stability and resistance properties of these materials.
- Pharmaceutical Manufacturing: Hydrogenation is employed in the pharmaceutical industry for synthesizing specific drug compounds or reducing functional groups in drug intermediates.
- Environmental Remediation: In some cases, hydrogenation is used for environmental purposes, such as the remediation of contaminated soil and water. It can help convert hazardous chemicals into less harmful forms.
- Chemical Research: Researchers often use hydrogenation in the laboratory to study reaction mechanisms, explore the properties of compounds, or develop new chemical processes.
- Industrial Lubricants and Cosmetics: Hydrogenation is used in the production of industrial lubricants and cosmetics to enhance their stability and performance.
The exact conditions and methods for hydrogenation can vary widely depending on the specific application. It may involve different catalysts, reaction temperatures, pressures, and equipment to achieve the desired results. Moreover, as environmental and sustainability concerns become more important, there is ongoing research and development in the field of hydrogenation to make the process more efficient and environmentally friendly.
Where is Required Hydrogenation
Hydrogenation is required and used in various industries and applications around the world. Here are some common places and contexts where hydrogenation is necessary or beneficial:
- Food Processing Facilities: Hydrogenation is extensively used in food processing plants to convert liquid vegetable oils into solid or semi-solid fats. This process is critical for the production of products like margarine, shortening, and various baked goods.
- Refineries: The petrochemical industry relies on hydrogenation in refineries to treat crude oil. It is used to reduce the levels of unsaturated hydrocarbons, ensuring the production of high-quality petroleum products such as gasoline, diesel, and jet fuel.
- Chemical Manufacturing Plants: Hydrogenation is a common operation in chemical manufacturing plants, where it is employed to synthesize a wide range of chemical compounds and intermediates, including pharmaceuticals, specialty chemicals, and industrial chemicals.
- Biodiesel Production Facilities: Biodiesel production facilities use hydrogenation to improve the stability and properties of biodiesel fuels. Unsaturated components in vegetable oils and animal fats are hydrogenated to meet quality standards.
- Polymer Production Plants: Hydrogenation is required in polymer production facilities, particularly in the production of elastomers and plastics. It helps modify the properties of these materials for specific applications.
- Pharmaceutical Laboratories: In pharmaceutical research and development, hydrogenation is a common technique used to synthesize and modify drug compounds.
- Environmental Cleanup Sites: Hydrogenation can be applied in environmental cleanup efforts to remediate contaminated soil and groundwater by reducing or transforming hazardous substances into less harmful forms.
- Chemical Research Laboratories: Researchers in academia and industry utilize hydrogenation in chemical research to investigate reaction mechanisms, explore the properties of compounds, and develop new chemical processes.
- Industrial Facilities Producing Lubricants and Cosmetics: Hydrogenation is used in the production of industrial lubricants, cosmetics, and personal care products to enhance their stability and performance.
- Energy Production: In some hydrogen-based energy production systems, hydrogenation is used as a method to store and release hydrogen gas for energy generation.
The need for hydrogenation varies depending on the specific industry, application, and product requirements. Different industries may use different catalysts, reaction conditions, and equipment to achieve their desired outcomes. The importance of hydrogenation in these industries highlights its versatile role in various chemical processes and product manufacturing.
How is Required Hydrogenation
The process of hydrogenation is conducted by carefully controlling the reaction conditions to ensure the desired outcomes, which can vary depending on the specific application and industry. Here’s a general overview of how hydrogenation is carried out:
- Selection of Reactants: First, the appropriate reactants are chosen. These reactants typically include an unsaturated compound that contains carbon-carbon double or triple bonds, such as unsaturated hydrocarbons or organic molecules with unsaturated functional groups.
- Choice of Catalyst: A suitable catalyst is selected to facilitate the hydrogenation reaction. Common catalysts used for hydrogenation include metals like nickel (Ni), palladium (Pd), platinum (Pt), or other transition metals. The choice of catalyst depends on the reactants and the reaction conditions.
- Reaction Vessel: The reaction takes place in a specialized reaction vessel or reactor. This vessel is designed to withstand the high pressures and temperatures often associated with hydrogenation. It may have a mechanism to introduce hydrogen gas into the reaction mixture.
- Hydrogen Gas Supply: Hydrogen gas (H2) is supplied to the reaction vessel. The hydrogen gas is typically purified to remove impurities that could interfere with the reaction.
- Control of Temperature and Pressure: Hydrogenation reactions are conducted at elevated temperatures and pressures, which can vary depending on the specific reactants and catalyst used. These conditions are carefully controlled to promote the reaction while ensuring safety.
- Stirring or Mixing: To ensure uniform mixing of the reactants and catalyst and to maximize the contact between the reactants and the hydrogen gas, stirring or mixing is often employed within the reaction vessel.
- Monitoring and Control: The progress of the hydrogenation reaction is monitored to determine when it is complete. This can be done through various analytical techniques, including gas chromatography, spectroscopy, or simply by observing changes in the reactants.
- Product Separation: Once the hydrogenation reaction is complete, the products are separated from the reaction mixture. This may involve filtration, distillation, or other separation techniques, depending on the specific compounds involved.
- Product Purification: In some cases, additional purification steps may be required to isolate the desired product in its purest form.
- Product Utilization: The hydrogenated product can be used in various industrial applications, depending on its intended purpose. For example, in the food industry, hydrogenated oils and fats may be used to produce margarine or shortenings.
It’s important to note that the specific conditions, catalysts, and procedures used in hydrogenation can vary widely depending on the reactants and the desired outcome. Additionally, safety precautions are crucial when working with hydrogen gas, as it is flammable and can pose safety risks if not handled properly. Industrial processes may involve large-scale hydrogenation operations, while laboratory-scale hydrogenation is used for research and development purposes.
Case Study on Hydrogenation
The Use of Hydrogenation in the Food Industry
Background: A large food processing company, ABC Foods Inc., specializes in the production of various food products, including margarine, baked goods, and snack items. ABC Foods has been facing challenges related to the texture, shelf life, and overall quality of its food products made with vegetable oils. The company has been using liquid vegetable oils in its products but is looking to create products with improved stability and consistency at room temperature.
Problem: ABC Foods Inc. is encountering issues with the texture and stability of its products made with liquid vegetable oils. The products tend to be too soft and have a shorter shelf life due to the oils’ susceptibility to oxidation. The company is also looking to produce solid fats for use in bakery applications.
Solution: To address these challenges, ABC Foods Inc. decides to implement the hydrogenation process. The goal is to hydrogenate the liquid vegetable oils used in their products to convert them into solid or semi-solid fats. This will enhance the texture, stability, and shelf life of their food products while also allowing for the production of various baked goods that require solid fats.
Implementation:
- Selection of Reactants: ABC Foods Inc. selects the appropriate vegetable oil as the reactant for hydrogenation. Common options include soybean oil, canola oil, or palm oil, which contain unsaturated fatty acids.
- Choice of Catalyst: The company selects a suitable hydrogenation catalyst based on their specific needs and equipment. They opt for a nickel (Ni) catalyst, which is commonly used in food-grade hydrogenation processes.
- Reaction Vessel: ABC Foods Inc. invests in specialized reaction vessels equipped with safety features to withstand high pressures and temperatures associated with hydrogenation.
- Hydrogen Gas Supply: The company ensures a reliable supply of pure hydrogen gas to be introduced into the reaction vessel. Safety measures are implemented to handle hydrogen gas safely.
- Control of Temperature and Pressure: The hydrogenation process is carried out at controlled temperatures and pressures, allowing for the gradual addition of hydrogen gas to the reactant.
- Stirring or Mixing: To maximize the contact between the reactant and the hydrogen gas, the reaction mixture is stirred or agitated continuously.
- Monitoring and Control: ABC Foods Inc. monitors the progress of the hydrogenation reaction through various analytical techniques. The reaction is allowed to continue until the desired degree of hydrogenation is achieved.
- Product Separation: After the reaction is complete, the hydrogenated fats are separated from the reaction mixture using filtration or other separation methods.
- Product Utilization: The hydrogenated fats produced are used in various food products, including margarine, shortening, and baked goods. These products now have improved texture and longer shelf life, meeting the company’s quality and stability requirements.
Results: As a result of implementing hydrogenation, ABC Foods Inc. achieves several benefits:
- Improved Texture: The hydrogenated fats have a more desirable texture, making the food products more appealing to consumers.
- Enhanced Shelf Life: The hydrogenation process reduces the susceptibility of fats to oxidation, extending the shelf life of the products.
- Expanded Product Range: The company can now produce a wider range of food products, including those that require solid fats, such as pastries and cookies.
- Consumer Satisfaction: Customers are more satisfied with the improved quality and consistency of ABC Foods Inc.’s food products.
Conclusion: By incorporating hydrogenation into their production process, ABC Foods Inc. successfully addresses the challenges related to the texture, stability, and quality of their food products. This case study highlights the versatility of hydrogenation in the food industry, where it plays a crucial role in improving the properties of various food items.
White paper on Hydrogenation
Transforming Chemistry for Industry and Beyond
Abstract:
- Summarize the content and importance of the white paper.
- Highlight the significance of hydrogenation in various industries and applications.
Table of Contents:
- Introduction to Hydrogenation:
- Define hydrogenation and its significance in the chemical industry.
- Briefly introduce the historical background and development of hydrogenation processes.
- Principles of Hydrogenation:
- Explain the fundamental chemistry behind hydrogenation reactions.
- Describe the role of catalysts and reaction conditions in facilitating hydrogenation.
- Discuss the difference between partial and complete hydrogenation.
- Hydrogenation in the Food Industry:
- Explore the critical role of hydrogenation in food processing.
- Explain how hydrogenation transforms liquid vegetable oils into solid fats for food products.
- Discuss the challenges and concerns related to trans fats in partially hydrogenated oils.
- Hydrogenation in Petrochemical Refining:
- Describe the application of hydrogenation in refining crude oil.
- Explain how hydrogenation reduces the level of unsaturated hydrocarbons and improves fuel quality.
- Hydrogenation in the Chemical Industry:
- Highlight the versatile use of hydrogenation in chemical synthesis.
- Provide examples of chemicals and pharmaceuticals synthesized through hydrogenation.
- Environmental and Sustainability Aspects:
- Discuss the environmental impact of hydrogenation processes.
- Explore efforts to make hydrogenation more sustainable, such as green catalysts and cleaner technologies.
- Challenges and Safety Considerations:
- Identify common challenges and safety issues associated with hydrogenation.
- Explain safety measures and best practices for handling hydrogen gas.
- Future Trends and Innovations:
- Analyze emerging trends in hydrogenation, such as the use of renewable hydrogen sources.
- Discuss ongoing research and innovation in the field.
- Conclusion:
- Summarize the key takeaways from the white paper.
- Emphasize the continued importance of hydrogenation in various industries and its role in shaping the future.
- References:
- Cite relevant sources and references used throughout the white paper.
Appendices:
- Include supplementary materials, such as detailed chemical reactions, case studies, or technical data, if applicable.
Acknowledgments:
- Acknowledge contributors, organizations, or sources of support, if necessary.
Remember to tailor the white paper to your specific audience and purpose, whether it’s for industry professionals, policymakers, researchers, or the general public. Additionally, you may want to include visuals, charts, and diagrams to enhance the understanding of the content.